|
Case Report
Vanadium allergy after bilateral total knee arthroplasty: Case report outcome of bilateral revision TKA
1 Harvard Medical School, Boston, MA, USA
2 Massachusetts General Hospital, Boston, MA, USA
Address correspondence to:
Matthew C Johnson
55 Fruit Street, YAW 3700, Boston, MA,
USA
Message to Corresponding Author
Article ID: 100025Z14MJ2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Johnson MC, Robinson MG, Siliski JM. Vanadium allergy after bilateral total knee arthroplasty: Case report outcome of bilateral revision TKA. J Case Rep Images Orthop Rheum 2021;5:100025Z14MJ2021.ABSTRACT
Introduction: Delayed metal hypersensitivity reactions following total knee arthroplasty (TKA) are a poorly understood phenomenon. Their relatively low incidence and insidious presentation make metal allergy a difficult and often-delayed diagnosis. Yet, metal allergies cause significant morbidity and decreased quality of life to the patients who are afflicted by them.
Case Report: We report the case of a 57-year-old woman with progressive erythema, dystonia, and pain after initially uncomplicated bilateral TKA for osteoarthritis. No signs of low-grade infection or mechanical causes of implant failure were present, and extensive workup—including initial 72-hour patch testing—was negative. Ultimately, repeat skin patch testing at 96 hours revealed an allergy to vanadium—a material present in both TKA tibial components. Nonoperative management failed to stop progression of symptoms.
Conclusion: Bilateral revision surgery was performed with cobalt chrome femoral and tibial components free of vanadium, and post-operative evaluations at 1 and 3 months showed outstanding responses. This case illustrates the importance of considering metal allergy screening as part of preoperative care in total joint replacement and provides clinical decision-making guidance on the novel case of vanadium allergy-induced TKA failure.
Keywords: Allergy, Arthroplasty, Revision, Vanadium
Introduction
As total joint replacement becomes exceedingly popular, cutaneous and systemic hypersensitivity reactions to implanted metals in total knee arthroplasty (TKA) are gathering increasing interest as a cause of implant failure. Metal allergy in the general population is quite common (10–20% of people) [1]. The most common culprits are nickel (14–19%) and cobalt (3–7%) with less prevalent causes being beryllium, titanium, and vanadium [2],[3]. In post-total joint replacement populations, the prevalence of metal allergy is even higher (25%), which is believed to be the result of prosthetic-induced sensitization [4]. In contrast, clinically significant deep tissue reactions to implants are relatively rare, affecting less than 1% of patients who undergo TKA [5]. Nonetheless, delayed-type metal hypersensitivities decrease functional outcomes and are a significant cause of morbidity for patients who are affected by them.
Metal allergy is believed to be a type IV (delayed-type) hypersensitivity reaction, and the known pathophysiology has previously been described in-depth [1],[6]. Briefly, metal corrosion generates ion haptens that complex with proteins. These metal-protein antigens are engulfed by histiocytes, which activate T lymphocytes. In response, a surge of proinflammatory cytokines is released, perpetuating immune cell recruitment and eliciting an immune response against the metal alloy. The resulting inflammatory milieu promotes bone resorption and can contribute to significant tissue damage, prosthetic loosening, and prosthetic failure [7]. Total knee arthroplasty implants are known to be particularly susceptible to high levels of corrosion and subsequent ion release [8].
The clinical presentation of metal allergy most frequently includes diffuse eczematous dermatitis, persistent pain, stiffness, and swelling of the area surrounding the replaced joint [9]. Often, these symptoms are difficult to distinguish from post-operative infection and mechanical complications. Thus, metal hypersensitivity reaction is a diagnosis of exclusion, considered only after eliminating the more typical causes of joint failure. Moreover, since no accepted diagnostic parameters exist, diagnosis is often difficult and delayed. For patients who fail nonoperative treatment, there is little published guidance on surgical decision-making and outcomes of revision surgery.
In our literature search, we found one case of vanadium allergy following TKA that was managed medically with topical corticosteroids [10]. We present the interesting case in which oral corticosteroids were attempted without improvement and revision surgery was undertaken. To our knowledge this is a novel treatment, since TKA due to vanadium allergy is exceedingly rare.
Case Report
A 57-year-old woman with a several year history of non-traumatic bilateral knee osteoarthritis presented for an orthopedic consult after progressive knee pain despite nonoperative treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroid injections. Her family history is notable for a brother with vanadium allergy following a hip replacement. She also had a suspected allergic reaction (although no laboratory studies were formed) following left wrist scaphoidectomy and 4 corner fusion, and she responded excellently to hardware removal. Radiographic evidence showed severe patellofemoral bone-on-bone osteoarthritis, and she elected to undergo bilateral TKA, starting with the right knee, followed by the left two months later. Smith and Nephew cruciate-retaining legion femoral components with Genesis II titanium alloy tibial baseplates were used for both knees (Table 1).
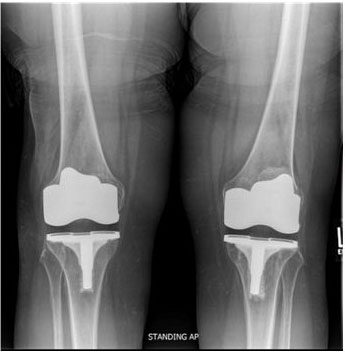
Her intra- and short-term postoperative course for the right TKA was uneventful: radiographs revealed a well fixed and aligned implant, and she had good incision healing and pain control with steady physical therapy progress. The only finding was mild right ankle burning pain at one-month post-op, considered to be routine. Her left TKA hospitalization was similarly uncomplicated; however, the patient developed post-operative redness on her left foot that progressed up her leg and persisted. At routine 12-week follow-up, the patient reported occasional discoloration and burning sensation of her left knee. Physical exam showed a diffuse erythematous/violaceous patch covering the entire lower extremity. She later developed knee pain on the right as well. Repeat radiographs revealed appropriate alignment with no signs of loosening (Figure 1).
Over the next 26 months the etiology of this patient’s pain remained unclear and perplexing. Workup for deep vein thrombosis, diabetes mellitus, B12 deficiency, chronic regional pain syndrome, and neuromuscular disorder was all negative. Arthrocentesis showed no signs of prosthetic joint infection and patch testing for metal allergy—including titanium and vanadium—was considered negative at 72 hours of testing. Given the low concern for aseptic loosening, scintigraphy was deferred.
Several trials of Botox, up-titrated pain control, foot massages, and oral prednisone bursts were attempted with only temporary palliation. Her mobility continued to markedly decline: her bilateral knee pain, skin redness, and cutaneous sensitivity steadily worsened, and she developed left foot cramping, involuntary left toe curling, foot inversion, back pain, and difficulty ambulating.
Metal hypersensitivity, despite a previous negative patch result, was reconsidered since no other cause of the patient’s pain could be identified. Ultimately, 96-hour patch testing revealed a positive reaction to vanadium—a metal contained in both total knee prosthetics as well as the hardware previously in her wrist.
Considering the patient’s steady deterioration despite oral prednisone trials as well as the success of wrist hardware removal in treating her previous metal hypersensitivity reaction, we recommended operative intervention. Bilateral knee revision surgeries of the implants containing vanadium were performed with DePuy Sigma posterior stabilized design with a cobalt chrome rotating platform tibial tray (Table 1).
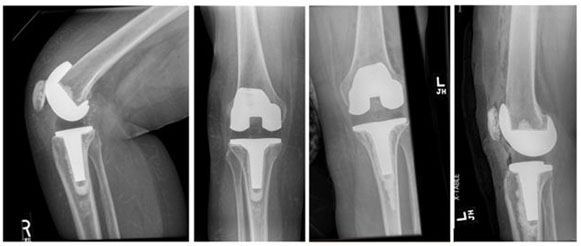
In both procedures, we utilized the previous midline anterior skin incision and medial parapatellar arthrotomy. The inflamed, fibrotic synovium was debrided and sent to pathology. Our concern for periprosthetic joint infection was low given clear joint fluid and low inflammatory markers, and we did not pursue fungal workup or send additional cultures. The femoral and tibial components were removed with minimal blood loss. Synovial biopsies were found to have wear debris, synovial fibrosis, and a histiocytic reaction, consistent with a type IV allergic reaction. Polyethylene articular surfaces were examined as a potential source of wear debris and showed unusually high wear at only two years (Figure 2). The new Sigma PS components were cemented into place, and the postoperative radiographs demonstrated excellent alignment (Figure 3).
In both cases, TKA revisions were completed without known complications and she went home on day of surgery. At both one- and three-month post-operative evaluations, she showed excellent recovery. Her range of motion increased bilaterally to 0–130 degrees from 0–105 degree before the TKA revisions. At three months postoperative, her mobility continues to improve, and she ambulates without gait abnormality. Her skin rash and sensitivity and her foot dystonia have also completely resolved. She still has persistent nerve-type pain in her toes but reports that is improving with time.
Discussion
Metal hypersensitivity after TKA remains a poorly understood cause of implant failure due to its rare occurrence and ambiguous clinical presentation. More common causes of prosthetic failure following TKA include infection (26–36%), instability (10–24%), loosening (17–22%), and arthrofibrosis (14–15%) [11],[12],[13]. In the setting of post-surgical complications, these etiologies should be investigated first and metal allergy only considered after ruling out the more likely reasons for TKA failure. Thus, identification of metal allergy requires a lengthy, sequential, and thorough workup. Due to these diagnostic challenges, clinicians must have high clinical suspicion to prevent delay in the identification and treatment of metal allergy and the adverse sequelae associate with it. Several authors have already identified the lack of accepted clinical parameters as an impediment to accurate and timely diagnosis of metal allergy [5],[14].
Recently, Flierl et al. reviewed the approach to painful TKA and provided a detailed algorithmic workup to correctly identify the cause of pain [15]. Allergy testing is most commonly assessed by patch testing, histology, and lymphocyte transformation tests (LTTs), and their combined approach has been recently summarized by Thomas et al. [16]. Importantly, due to the high prevalence of metal allergy and the low incidence of hypersensitivity-induced complications, positive patch testing alone does not prove causality [17]. Diagnosis should be supported by histological findings consistent with hypersensitivity reaction, and final interpretation, of course, must take the clinical picture into account [9].
In our case, extensive workup for common causes of implant complications was completed without identifying the cause of the patient’s symptoms. The patient’s past surgical history raised suspicion for metal allergy; however, initial patch testing at 72 hours was not sensitive enough to identify her allergy—and a false negative delayed her diagnosis by 15 months. Our case highlights the need for diligence in performing long-duration allergy testing for patients with expected metal hypersensitivity. The low sensitivity of short-term patch testing—especially for metal allergy—is already well-documented in the literature [18],[19]. The European Society of Contact Dermatitis’s best practice recommendation is that patch testing should not be considered negative until there is documented lack of reaction on either day 2, 3, or 4 and on day 7 [20]. This guideline was recently supported by a large, retrospective data analysis that found 13.6% of patients showed new positive patch testing results on day 7 as compared to day 3 [21]. The initial patch testing of our patient illustrates this point well: short-term negative patch does not rule-out metal allergy; long-term incubation is required.
Due to its low cost and accessibility, patch testing remains the gold standard for the diagnosis of suspected metal hypersensitivity. However, LTT is an alternative option that may be particularly helpful in cases of equivocal patch testing results in the setting of high allergy suspicion [22]. Lymphocyte transformation test works by measuring the proliferation of lymphocytes after exposure to a suspected allergen and comparing that rate to the expected non-allergenic basal rate. This test is being increasingly utilized in conjunction with patch testing, and its diagnostic use in a difficult case of TKA metal allergy was recently highlighted by Lieberman et al. [23].
The relationship between aseptic joint loosening and allergy sensitization is an exciting and controversial clinically relevant topic. The current prevailing idea is that metal ions, released by corrosion, elicit immune response activation and cause joint loosening. Several studies have demonstrated that metal implants undergo corrosion in vivo and metal particles can be found locally as well as widely disseminated throughout the body in patients post-arthroplasty [24],[25],[26]. However, it remains to be understood whether metal allergy is the cause or result of joint loosening. Less commonly discussed is the role of polyethylene inserts in metal allergy. Polyethylene wear debris is already recognized as a significant contributor to aseptic loosening and decreased prosthetic duration; however it also prevents metal-on-metal contact and decreases metal ion generation [27],[28]. Thus, polyethylene has both protective and risk factors for metal allergy. Yet, a role for polyethylene wear in metal allergy has not been systematically studied or characterized. In this case, we noticed accelerated polyethylene articular surface wear, which raises questions about joint life duration in TKA patients with persistent metal allergy who do not undergo revision surgery [29]. Nevertheless, we recognize the limitation that this report is only single case of an uncommon allergy, and further research is necessary.
In cases of metal implant hypersensitivity, the first line treatment is typically a 3-week trial of topical corticosteroids applied over the affected area [30]. Previously, Peat et al. published a case report of a patient with a cutaneous allergy to vanadium after TKA managed non-operatively using this approach [10]. They reported partial response to topical steroids. Likewise, we also attempted initial treatment with corticosteroids; however, our patient did not respond as favorably and required revision surgery. In general, revision surgery is a more effective treatment and is considered curative [30]. Nevertheless, due to its low prevalence, there has been no published data on revision surgery for vanadium allergy following TKA.
In their paper, Peat et al. recognized that TKA revision surgery for vanadium hypersensitivity would be challenging due to the difficulty of finding a TKA prosthetic without vanadium. We report this new case to identify a TKA prosthetic compatible with vanadium allergy and describe the surgical management relevant in this situation. While most TKA tibial components are titanium alloy, which contains vanadium, we utilized a cobalt-chromium tibial tray free of vanadium for both revision TKAs. Several TKA implant companies have vanadium-free cobalt-chrome tibial tray implant options. Most companies will also offer custom implants to avoid certain metals if a patient is allergic to a particular metal in the titanium alloy. Nevertheless, particular attention should be paid to component removal during revision surgery, as significant bone loss could require the use of augments or metaphyseal sleeves, which are often composed of titanium alloy. These data illustrate that revision surgery to components without vanadium is a promising solution to TKA patients with vanadium allergy with persistent cutaneous and systemic symptoms.
Conclusion
Metal hypersensitivity reaction is an incompletely understood and uncommon cause of TKA failure; however, it is increasingly recognized as a potential contributor to adverse post-operative outcomes. Here, we review the diagnostic workup of metal allergy and report the pitfall of short-term patch testing. Previous work has described the nonoperative management of vanadium allergy following total knee arthroplasty; however, there have been no reports of vanadium allergy requiring revision after TKA. Our case identifies revision TKA as an effective treatment option for patients with vanadium allergy who fail nonoperative management, and we outline the orthopedic clinical decision-making and outcomes relevant in this circumstance.
REFERENCES
1.
Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am 2001;83(3):428–36. [CrossRef]
[Pubmed]

2.
Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol 2010;23(2):309–18. [CrossRef]
[Pubmed]

3.
Uter W, Werfel T, White IR, Johansen JD. Contact allergy: A review of current problems from a clinical perspective. Int J Environ Res Public Health 2018;15(6):1108. [CrossRef]
[Pubmed]

4.
Niki Y, Matsumoto H, Otani T, Yatabe T, et al. Screening for symptomatic metal sensitivity: A prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials 2005;26(9):1019–26. [CrossRef]
[Pubmed]

5.
Bao W, He Y, Fan Y, Liao Y. Metal allergy in total-joint arthroplasty: Case report and literature review. Medicine (Baltimore) 2018;97(38):e12475. [CrossRef]
[Pubmed]

6.
Roberts TT, Haines CM, Uhl RL. Allergic or hypersensitivity reactions to orthopaedic implants. J Am Acad Orthop Surg 2017;25(10):693–702. [CrossRef]
[Pubmed]

7.
Hallab NJ, Vermes C, Messina C, Roebuck KA, Glant TT, Jacobs JJ. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res 2002;60(3):420–33. [CrossRef]
[Pubmed]

8.
Lützner J, Dinnebier G, Hartmann A, Günther KP, Kirschner S. Study rationale and protocol: Prospective randomized comparison of metal ion concentrations in the patient’s plasma after implantation of coated and uncoated total knee prostheses. BMC Musculoskelet Disord 2009;10:128. [CrossRef]
[Pubmed]

9.
Thomas P. Clinical and diagnostic challenges of metal implant allergy using the example of orthopaedic surgical implants: Part 15 of the series molecular allergology. Allergo J Int 2014;23(6):179–85. [CrossRef]
[Pubmed]

10.
Peat F, Coomber R, Rana A, Vince A. Vanadium allergy following total knee arthroplasty. BMJ Case Rep 2018;2018:bcr2017222092. [CrossRef]
[Pubmed]

11.
Pitta M, Esposito CI, Li Z, Lee YY, Wright TM, Padgett DE. Failure after modern total knee arthroplasty: A prospective study of 18,065 knees. J Arthroplasty 2018;33(2):407–14. [CrossRef]
[Pubmed]

12.
Postler A, Lützner C, Beyer F, Tille E, Lützner J. Analysis of Total Knee Arthroplasty revision causes. BMC Musculoskelet Disord 2018;19(1):55. [CrossRef]
[Pubmed]

13.
Abdel MP, Ledford CK, Kobic A, Taunton MJ, Hanssen AD. Contemporary failure aetiologies of the primary, posterior-stabilised total knee arthroplasty. Bone Joint J 2017;99-B(5):647–52. [CrossRef]
[Pubmed]

14.
Lachiewicz PF, Watters TS, Jacobs JJ. Metal hypersensitivity and total knee arthroplasty. J Am Acad Orthop Surg 2016;24(2):106–12. [CrossRef]
[Pubmed]

15.
Flierl MA, Sobh AH, Culp BM, Baker EA, Sporer SM. Evaluation of the painful total knee arthroplasty. J Am Acad Orthop Surg 2019;27(20):743–51. [CrossRef]
[Pubmed]

16.
Thomas P, Summer B, Thyssen JP. Hypersensitivity Reactions to Orthopedic Implants. In: Johansen JD, Mahler V, Lepoittevin JP, Frosch PJ, editors. Contact Dermatitis, Cham: Springer International Publishing; 2020. p. 1–9. [CrossRef]

17.
Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: A perspective from The American Contact Dermatitis Society. Dermatitis 2016;27(5):241–7. [CrossRef]
[Pubmed]

18.
Higgins E, Collins P. The relevance of 7-day patch test reading. Dermatitis 2013;24(5):237–40. [CrossRef]
[Pubmed]

19.
Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: An update from the Mayo Clinic Contact Dermatitis Group. Dermatitis 2017;28(4):253–60. [CrossRef]
[Pubmed]

20.
Johansen JD, Aalto-Korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing – Recommendations on best practice. Contact Dermatitis 2015;73(4):195–221. [CrossRef]
[Pubmed]

21.
van Amerongen CCA, Ofenloch R, Dittmar D, Schuttelaar MLA. New positive patch test reactions on day 7 – The additional value of the day 7 patch test reading. Contact Dermatitis 2019;81(4):280–7. [CrossRef]
[Pubmed]

22.
Richards LJ, Streifel A, Rodrigues JM. Utility of patch testing and lymphocyte transformation testing in the evaluation of metal allergy in patients with orthopedic implants. Cureus 2019;11(9):e5761. [CrossRef]
[Pubmed]

23.
Lieberman EG, Barrack RL, Schmalzried TP. Suspected metal allergy and femoral loosening after total knee arthroplasty: A diagnostic dilemma. Arthroplast Today 2021;7:114–9. [CrossRef]
[Pubmed]

24.
Singh R, Dahotre NB. Corrosion degradation and prevention by surface modification of biometallic materials. J Mater Sci Mater Med 2007;18(5):725–51. [CrossRef]
[Pubmed]

25.
Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening—Current concepts. J Biomed Mater Res A 2009;91(4):1252–62. [CrossRef]
[Pubmed]

26.
Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am 2000;82(4):457–76.
[Pubmed]

27.
Savarino L, Granchi D, Ciapetti G, et al. Ion release in patients with metal-on-metal hip bearings in total joint replacement: A comparison with metal-on-polyethylene bearings. J Biomed Mater Res 2002;63(5):467–74. [CrossRef]
[Pubmed]

28.
Gao X, He RX, Yan SG, Wu LD. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty 2011;26(4):665.e13–6. [CrossRef]
[Pubmed]

29.
Granchi D, Cenni E, Trisolino G, Giunti A, Baldini N. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater 2006;77(2):257–64. [CrossRef]
[Pubmed]

30.
Wawrzynski J, Gil JA, Goodman AD, Waryasz GR. Hypersensitivity to orthopedic implants: A review of the literature. Rheumatol Ther 2017;4(1):45–56. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Matthew C Johnson - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew G Robinson - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
John M Siliski - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Matthew C Johnson et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.